
Autor: David Grooms
Datum: 08.07.2019

Die Häufigkeit von Dyssynchronien wurde untersucht; gemäss Schätzungen tritt bei nicht weniger als 50 % der Patienten, die für mindestens 24 Stunden maschinell beatmet werden, mindestens einmal eine Dyssonchronie auf. Die zwei häufigsten Typen von Dyssynchronie sind ineffektive (erfolglose) Triggerung und Doppel-Triggerung (DT) (
Die wichtigste Methode zur Erkennung von DT ist die Beobachtung und Auswertung der skalaren Kurven des Beatmungsgerätes. Unter einer skalaren Kurve versteht man die Darstellung einer jeglichen Variable im zeitlichen Verlauf. Die meisten Beatmungsgeräte bieten für gewöhnlich die Darstellung von Druck, Flow und/oder Volumen im zeitlichen Verlauf. Für eine einfachere Analyse dieser Kurven ermöglichen manche Beatmungsgeräte zusätzlich die Darstellung des ösophagealen Drucks (Näherungswert für Pleuradruck) im zeitlichen Verlauf. Die Schritte für eine angemessene Erkennung von DT sind in Screenshots von Kurven am Beatmungsgerät unten angeführt. Abbildung 1 zeigt häufige Druck, Flow- und Volumenkurven, die das DT-Phänomen bei invasiver Beatmung darlegen. Für ein ungeschultes Auge ist es anfangs u. U. schwierig, dieses Phänomen zu erkennen und die Ursache dieses Problems korrekt zu bestimmen. Häufig wird fälschlicherweise angenommen, dass der Patient nach Verabreichung des maschinell ausgelösten Atemhubs (Atemhub 1) aktiv einen zweiten Atemhub getriggert hat (Atemhub 2) oder dass der Patient „lufthungrig“ ist. Wenn das Problem nicht behoben wird, kann es zu schwerwiegenden unerwünschten Wirkungen bei der maschinellen Beatmung kommen. Deswegen wird eine genauere Analyse empfohlen. Dazu kann eine Messung des ösophagealen Drucks vorgenommen werden, um den Pleuradruck mit dem Atemwegsdruck und den Flow-Veränderungen des Beatmungsgerätes zu vergleichen und zu beurteilen. In einem weiteren Beispiel unten werden auf einem Beatmungsgerät die Skalare für Druck und Flow im Verlauf der Zeit angezeigt. Dies bietet einen subtilen Hinweis auf eine mögliche DT, kann aber auch als zusätzliche aktive inspiratorischen Bemühung des Patienten missgedeutet werden (Abbildung 2). Nach dem Hinzufügen der skalaren Kurve für den ösophagealen Druck (Pes (Paux)-Kurve) wird klar, dass es sich in der Tat um Doppel-Triggerung handelt, da während einer einzigen aktiven inspiratorischen Bemühung ein weiterer Atemhub verabreicht wird (siehe Abnahme im Pleuradruck in Abbildung 3).
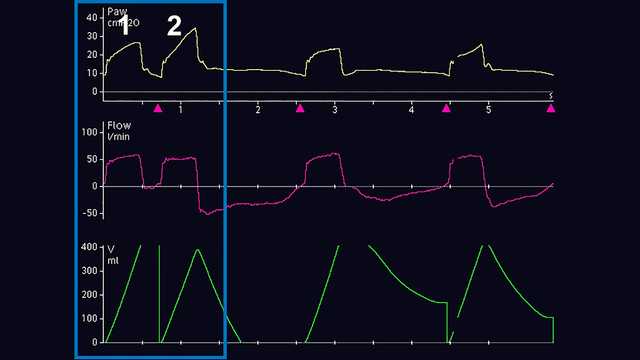
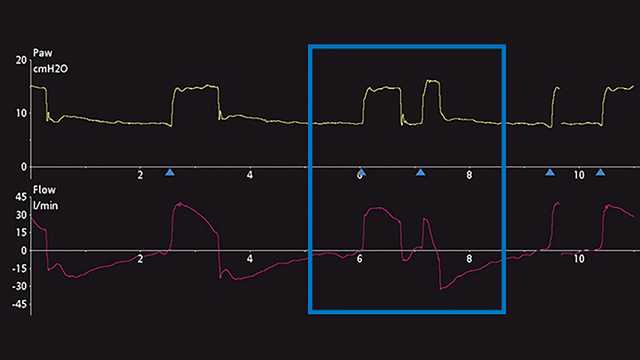
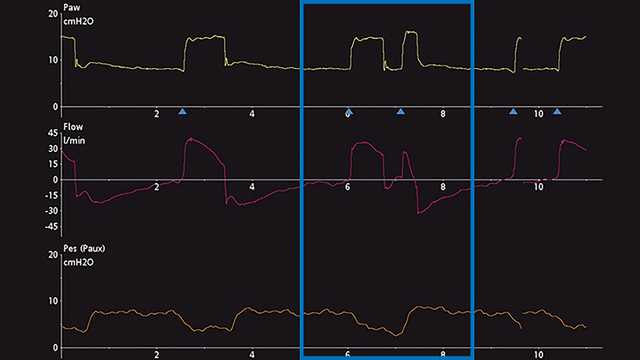
Den Typ der DT zu erkennen und einzuteilen, kann am Patientenbett auch eine Herausforderung darstellen. Gemäss aktuellen Studien kann die DT in drei verschiedene Typen unterteilt werden (
Daten belegen, dass es in der prä-inspiratorischen Phase oft zu einer Verzögerung der Triggerung von 0,07 bis 0,13 Sekunden kommt (
Die häufigsten Ursachen für DT sind eine mangelnde Abstimmung der Inspirationszeiten bei maschinellen Atemhüben mit neuralen Inspirationszeiten sowie eine unzureichende Druckunterstützung mit hohen Atemantrieben (