All Hamilton Medical ventilators – from high-end to transport devices – offer the option of noninvasive and invasive ventilation, with conventional and advanced ventilation modes for even the smallest patients.
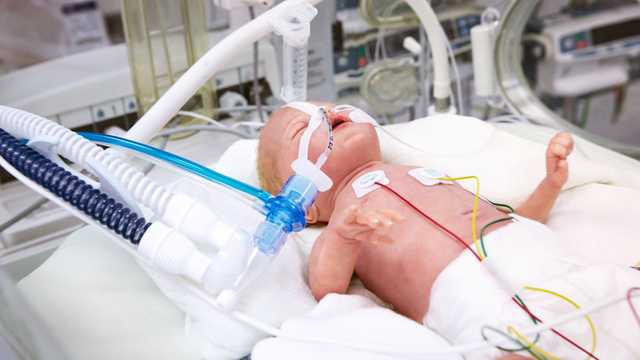
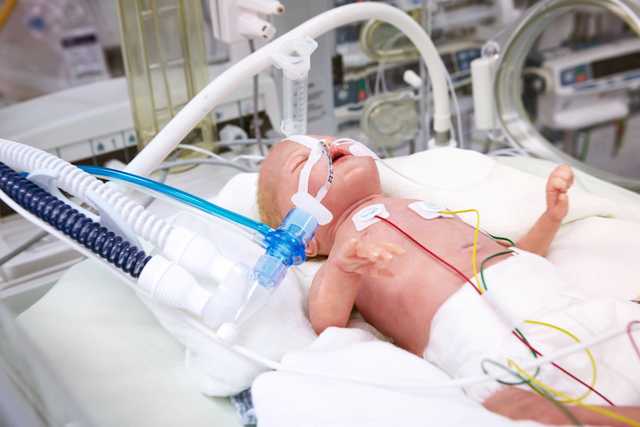
The proximal flow sensor and expiratory valve enable the precise measurement of pressure and flow directly at the airway opening, ensuring the required sensitivity and a quick response time.

With the neonatal option, our devices provide tidal volumes as low as 2 ml for effective, safe, and lung-protective ventilation for neonates (
The nCPAP modes are designed in such a way that you only need to set the desired CPAP/PEEP. The flow is subsequently adjusted automatically based on the patient need (demand flow) and variation in leakage, which prevents unintended peak pressures or loss of CPAP/PEEP, and guarantees leak compensation.
Book a free personal demonstration with one of our specialists to explore the benefits of our NICU ventilators further. They can guide you through the features, functionalities, and suitability of our devices based on your specific needs.
Alternatively, you can schedule a callback, and our team will reach out to provide you with the necessary information and assistance.