Unser intelligenter Beatmungsmodus befördert Sie vom Tastendrücker zum Entscheider. INTELLiVENT-ASV reduziert die Anzahl der manuellen Einstellungen am Beatmungsgerät (
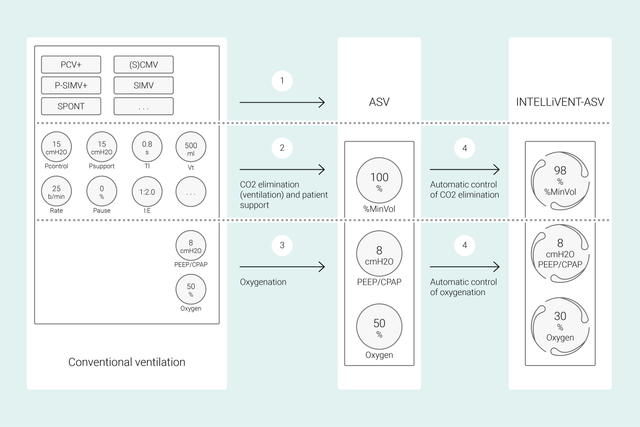
Bei konventionellen Beatmungsmodi stellen Sie mehrere Parameter am Beatmungsgerät ein (z. B. Tidalvolumen oder Druck, Atemfrequenz, FiO2, PEEP sowie die Exspirations- und Inspirationszeit), um bestimmte klinische Ziele zu erreichen. Alle diese Parameter müssen auch noch häufig neu beurteilt und angepasst werden.
Bei INTELLiVENT-ASV stehen dagegen die von Ihnen festgelegten klinischen Ziele und Strategien für die Oxygenierung und Beatmung im Mittelpunkt. Nachdem Sie diese Ziele eingestellt haben, entscheiden Sie, inwieweit INTELLiVENT‑ASV die Regelung der Oxygenierung und Beatmung übernehmen soll, um die Ziele zu erreichen.
INTELLiVENT-ASV wählt anschliessend die Beatmungseinstellungen automatisch aus, steuert den Übergang zwischen dem aktiven und passiven Zustand und unterstützt aktiv Ihre Entwöhnungsprotokolle mit Quick Wean.
Mehrere internationale Studien belegen die Sicherheit und Wirksamkeit von INTELLiVENT-ASV in verschiedenen klinischen Szenarien – nach Herzoperationen (


In diesem Video zeigt der Oberarzt der Intensivmedizin Dr. Jean-Michel Arnal die wichtigsten Funktionen und Einstellungen für den Modus INTELLiVENT-ASV an einem echten Patienten auf der Intensivstation.
Zuerst stellen Sie Grösse, Geschlecht und ggf. die bestimmten Zustände für den Patienten ein: ARDS, chronische Hyperkapnie oder Schädel-Hirn-Trauma. Danach legen Sie die klinischen Zielwerte für die Oxygenierung (SpO2) und CO2-Eliminierung (PetCO2) für den Patienten fest.
Nach diesen Einstellungen haben Sie verschiedene Möglichkeiten für die Feinabstimmung von INTELLiVENT-ASV. Sie können entscheiden, ob Sie den PEEP manuell festlegen möchten oder ob INTELLiVENT‑ASV den PEEP-Wert in einem von Ihnen definierten Bereich einstellen soll. Nachdem Sie die Alarmgrenzwerte geprüft und ggf. angepasst haben, können Sie die Beatmung starten.
INTELLiVENT‑ASV setzt Ihre Strategie am Patientenbett um. Anstatt immer wieder einzelne Einstellungen zu ändern, überwachen Sie die Zielwerte und passen die Strategie nur bei Bedarf an.
INTELLiVENT-ASV zielt darauf ab, dass die Zielwerte erreicht werden und im definierten Bereich bleiben – und stellt eine gleichbleibend lungenschonende Beatmung sicher (
Diese Daten werden von drei Sensoren gemessen: Der proximale Flow-Sensor stellt Daten zur Lungenmechanik und zur Spontanaktivität des Patienten bereit, während der SpO2-Sensor und der CO2-Sensor Informationen zur Oxygenierung und CO2-Eliminierung liefern.
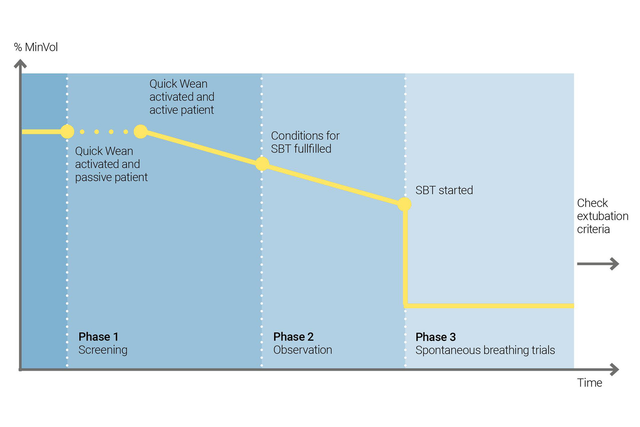
Nutzen Sie die Quick Wean-Funktion von INTELLiVENT-ASV, um Ihr Entwöhnungsprotokoll umzusetzen. Sie können Quick Wean während der Beatmung aktivieren, wenn der Patient spontan atmet.
Sie können Quick Wean konfigurieren, indem Sie durch einen SBT die Bereitschaft des Patienten für eine Trennung vom Beatmungsgerät bewerten lassen. Sie legen die Kriterien fest, wann ein SBT gestartet werden soll, welche Einstellungen während des SBT angewendet werden und wann der SBT abgebrochen wird.
INTELLiVENT-ASV zeigt immer eine Übersicht aller durchgeführten SBTs an. Ist ein SBT nicht erfolgreich, kehrt INTELLiVENT-ASV zu den vorherigen Einstellungen für die Beatmung zurück.
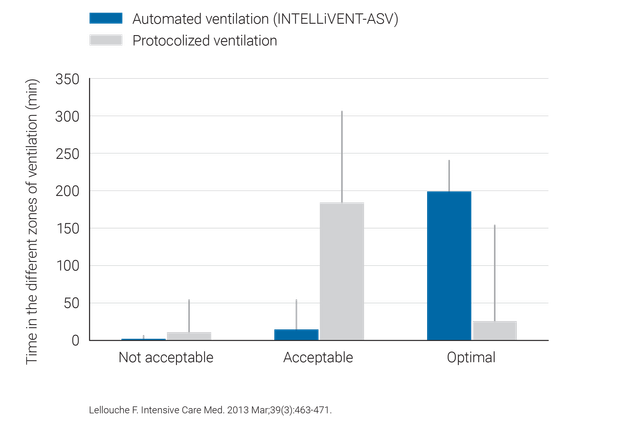
Klinische Studien belegen, dass INTELLiVENT-ASV sichere Einstellungen für Driving Pressure (
INTELLiVENT-ASV erfordert weniger manuelle Anpassungen als konventionelle Beatmungsmodi und trägt so zur Entlastung des Pflegepersonals bei (

INTELLiVENT-ASV ist auf den Beatmungsgeräten HAMILTON-G5, HAMILTON-C6, HAMILTON-C3, HAMILTON-C1 und HAMILTON-T1 als Option verfügbar und gehört auf dem HAMILTON-S1 zu den Standardmodi.