Autor: Munir Karjaghli, especialista en aplicaciones clínicas, Hamilton Medical AG
Fecha: 15.11.2021

La ventilación mecánica durante la ventilación unipulmonar tiene tres objetivos: (I) ayudar en la eliminación del dióxido de carbono, (II) mantener la oxigenación y (III) reducir la disfunción pulmonar posoperatoria. Se han llevado a cabo numerosos estudios para determinar la estrategia más adecuada para la ventilación mecánica durante la ventilación unipulmonar.
Hay muy diversos factores que pueden contribuir a la LPA perioperatoria. La lesión pulmonar se produce a raíz de la tensión mecánica provocada por la hiperinflación, hiperperfusión y reclutamiento/desreclutamiento cíclico, junto con factores proinflamatorios o bioquímicos. En el caso de los pacientes sometidos a cirugía torácica, una teoría de ‘varios resultados' sugiere que una combinación de factores relacionados con la cirugía, la ventilación unipulmonar, enfermedades subyacentes y morbilidades asociadas, el tratamiento anterior y otros acontecimientos sin identificar pueden provocar una mayor susceptibilidad a la LPA (
La ventilación unipulmonar durante y después de la cirugía torácica aumenta el riesgo de volutrauma, barotrauma, atelectrauma y toxicidad del oxígeno, todas ellas complicaciones graves que causan lesión pulmonar inducida por el respirador (
Existen muy pocos datos que respalden concretamente un enfoque específico del tratamiento de la ventilación unipulmonar en cuanto a resultados clínicos. La definición de lo que se considera ventilación unipulmonar con protección se ve influida principalmente por la opinión de expertos, las pruebas reunidas de la ventilación bipulmonar en pacientes sometidos a cirugía general y un escaso número de ensayos clínicos. Es muy difícil señalar el volumen tidal, por ejemplo, como único factor contribuyente de la lesión pulmonar durante la ventilación unipulmonar. En ningún estudio hasta la fecha se ha demostrado definitivamente ninguna ventaja específica de la ventilación con volumen tidal (Vt) bajo durante la ventilación unipulmonar en ausencia de otras estrategias respiratorias, como la presión positiva al final de la espiración (PEEP) (
En un estudio retrospectivo llevado a cabo después de implementar un protocolo de ventilación con protección durante la ventilación unipulmonar para cirugía de cáncer de pulmón, en el que se incluyó un Vt reducido, aumento de la PEEP, presiones del respirador limitadas y maniobras de reclutamiento, se descubrió menor riesgo de lesión pulmonar aguda (
La Society for Translational Medicine presenta recomendaciones basadas en las pruebas actuales para la ventilación unipulmonar en sus directrices de práctica clínica para el manejo de la ventilación mecánica en pacientes sometidos a lobectomía (
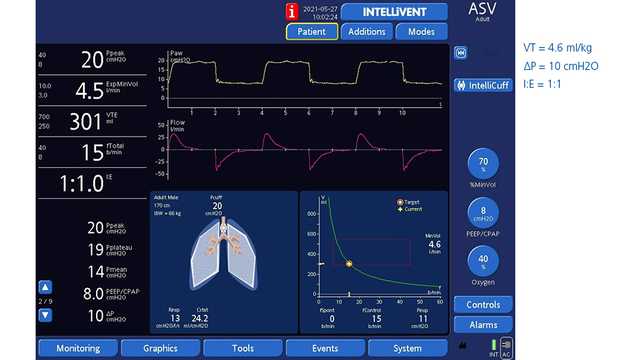
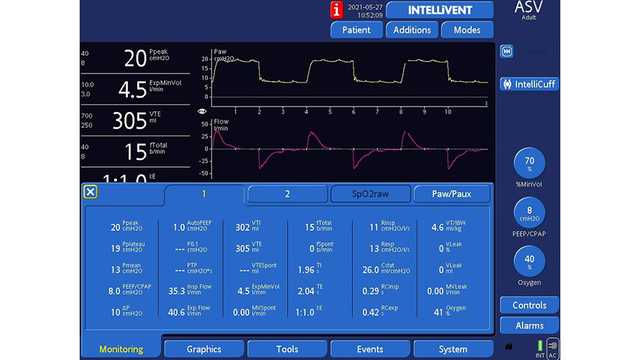
El modo de ventilación asistida adaptable (ASV®) en todos los respiradores de Hamilton Medical implementa automáticamente una estrategia de protección pulmonar que cumple las recomendaciones de volumen tidal y presión de trabajo en la ventilación unipulmonar. Además, el modo de bucle completamente cerrado INTELLiVENT®-ASV (
En un estudio de Weiler et al. se revela que la ASV puede suministrar ventilación a los pacientes de forma segura, incluso bajo las condiciones tan variables de la ventilación unipulmonar (
En las figuras 1 y 2 siguientes se muestra un paciente masculino de 61 años con ventilación ASV que se sometió a una neumonectomía derecha.
Puede consultar las citas completas a continuación: (