La nostra modalità di ventilazione intelligente permette di ridurre il tempo perso a girare manopole, trasformando l'operatore in un supervisore. INTELLiVENT-ASV riduce il numero di interazioni manuali con il ventilatore (
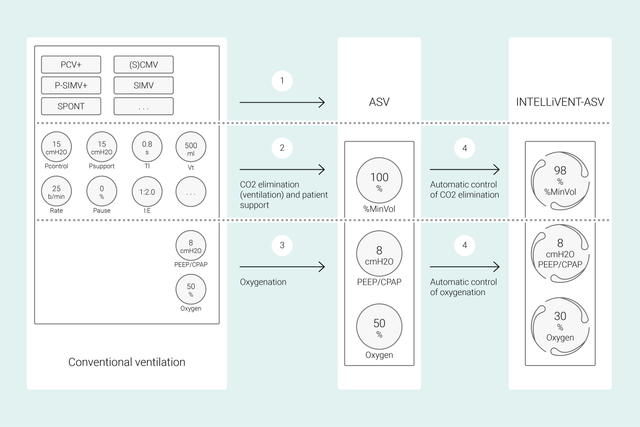
Con le modalità convenzionali, l'operatore imposta i diversi comandi del ventilatore, come volume corrente o pressione, frequenza respiratoria, FiO2, PEEP e tempi espiratorio e inspiratorio, per ottenere determinati obiettivi clinici. Le impostazioni di tutti questi comandi devono inoltre essere rivalutate e regolate di frequente.
Con INTELLiVENT-ASV, il lavoro è invece incentrato sugli obiettivi clinici e sulle strategie di ossigenazione e ventilazione definite dall'operatore. Dopo aver inserito questi obiettivi, è possibile decidere in che misura INTELLiVENT-ASV deve controllare ossigenazione e ventilazione per raggiungerli.
INTELLiVENT-ASV seleziona quindi automaticamente le impostazioni del ventilatore, gestisce la transizione fra stati passivi e attivi e supporta attivamente i protocolli di svezzamento stabiliti grazie alla funzione di Svezzamento veloce.
Diversi studi internazionali hanno dimostrato la sicurezza e le prestazioni di INTELLiVENT-ASV in svariate situazioni cliniche, dalla fase postoperatoria degli interventi cardiochirurgici (


In questo video, il Dr. Jean-Michel Arnal, intensivista esperto, fornisce una rapida dimostrazione delle principali funzioni e impostazioni di INTELLiVENT-ASV utilizzate su un paziente reale ricoverato in terapia intensiva.
Per prima cosa, si impostano altezza, sesso e, se necessario, eventuali condizioni specifiche del paziente: ARDS, ipercapnia cronica o danno cerebrale. Si impostano quindi gli obiettivi clinici per il paziente in termini di ossigenazione (SpO2) ed eliminazione della CO2 (PetCO2).
Sono inoltre disponibili diverse opzioni per la regolazione di precisione di INTELLiVENT-ASV. Per esempio, si può scegliere di impostare la PEEP manualmente o di farla impostare a INTELLiVENT-ASV rispettando i limiti di un intervallo definito dall'operatore. Dopo aver controllato o impostato i limiti di allarme, si può iniziare la ventilazione.
INTELLiVENT-ASV mette in pratica la vostra strategia restando al posto letto. Invece di dover modificare di frequente le singole impostazioni, dovrete monitorare e regolare i valori target solo quando è necessario.
INTELLiVENT-ASV cerca di portare il paziente all'interno dell'intervallo target definito dall'operatore e di mantenerlo entro i valori impostati, applicando sempre una ventilazione con protezione polmonare (
Questi dati vengono misurati da tre sensori: il sensore di flusso prossimale fornisce i dati sulla meccanica polmonare e l'attività del paziente, mentre i sensori di SpO2 e CO2 raccolgono dati sull'ossigenazione e sull'eliminazione della CO2.
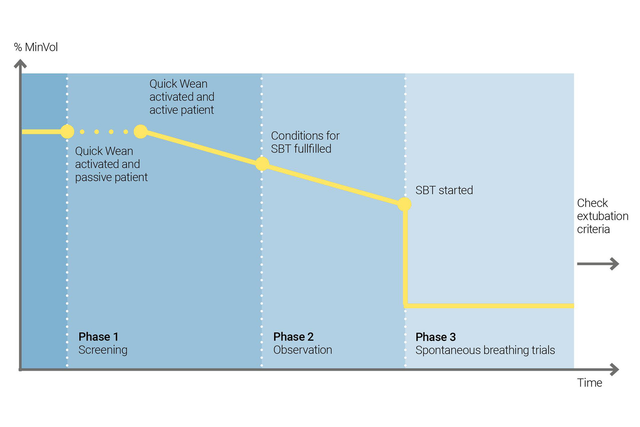
È possibile utilizzare la funzione di Svezzamento veloce di INTELLiVENT-ASV per mettere in pratica il vostro protocollo di svezzamento. Quando il paziente respira spontaneamente, si può abilitare la funzione di Svezzamento Veloce durante la ventilazione.
È quindi possibile configurare lo Svezzamento Veloce consentendo gli SBT per valutare se il paziente sia pronto al distacco dal ventilatore. l'operatore modifica i criteri di avvio degli SBT, le impostazioni da usare durante gli SBT e i criteri in base a cui interromperli.
INTELLiVENT-ASV visualizza sempre la cronologia di tutti gli SBT eseguiti. Se un SBT ha esito negativo, INTELLiVENT-ASV torna ad applicare le impostazioni di ventilazione precedenti.
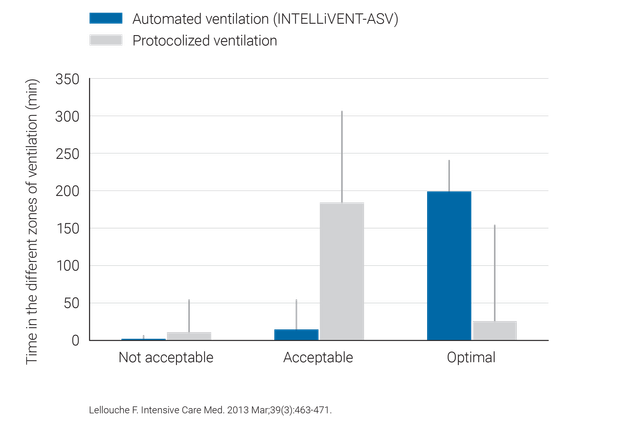
Alcuni studi clinici hanno dimostrato che INTELLiVENT-ASV sceglie una driving pressure sicura (
INTELLiVENT-ASV richiede un minor numero di regolazioni manuali rispetto alla ventilazione convenzionale, pertanto agevola la riduzione del carico di lavoro del personale sanitario (

INTELLiVENT-ASV è disponibile come opzione sui ventilatori HAMILTON-G5, HAMILTON-C6, HAMILTON-C3, HAMILTON-C1 e HAMILTON-T1; è invece una funzione standard su HAMILTON-S1.