Footnotes
- A. Para usuários nos EUA, o controlador USB Aerogen só deve ser operado com alimentação da rede elétrica usando o adaptador AC/DC do controlador USB Aerogen.
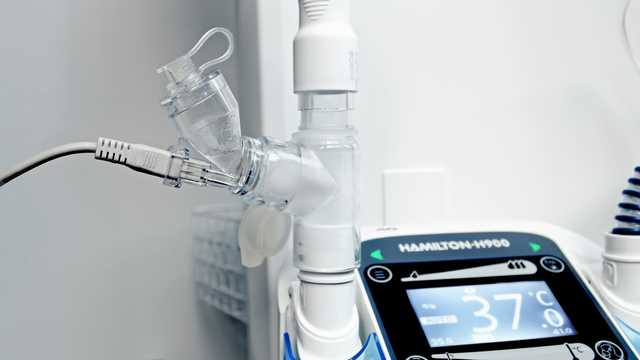
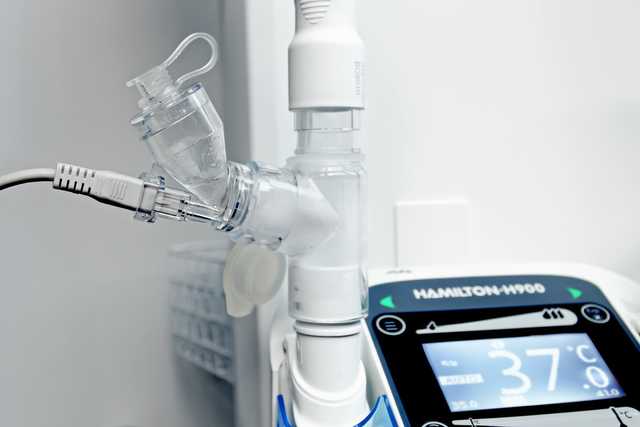
Oferecemos soluções de nebulização da Aerogen, fabricante e distribuidora líder mundial de tecnologia de alto desempenho para o tratamento com medicamentos em aerossol.

A tecnologia de malha vibratória produz partículas consistentes do tamanho de gotículas:

Em todo o hospital, o controlador Pro-X fornece energia para as tecnologias Aerogen em um dispositivo portátil.

O controlador de USB Aerogen de estrutura fina pode ser usado para alimentar o Aerogen Solo a partir da porta USB do seu respirador (