我们的智能通气模式能使您从操作者转变为监督者。INTELLiVENT-ASV 减少与呼吸机手动互动的次数 (
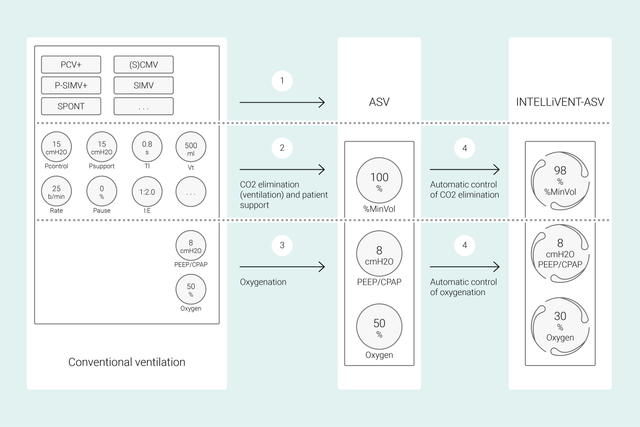
在常规模式下,您设置多个呼吸机控制参数(如潮气量或压力、呼吸频率、FiO2、PEEP 和呼气及吸气时间)来实现某些临床目标。此外,所有这些控制参数都需要频繁再评估和调节。
使用 INTELLiVENT-ASV,您所确定的临床目标和对氧合和通气的策略即为重点。设定上述目标后,您可以决定 INTELLiVENT-ASV 控制氧合和通气的程度范围,从而实现该目标。
然后 INTELLiVENT-ASV 自动选择呼吸机设置,管理被动和主动呼吸状态之间的转换,并利用快速撤机功能主动为您的撤机方案提供支持。
多项国际研究证明了 INTELLiVENT-ASV 在各种临床情景中的安全性和性能——从心脏手术后 (


在本视频中,高级医师 Jean-Michel Arnal 博士在真实 ICU 病人中为您快速演示了 INTELLiVENT-ASV 的主要功能和设置。
开始时,设置病人的身高、性别和(必要时)特定状况:ARDS、慢性高碳酸血症或脑损伤。接着,为病人设置氧合状态 (SpO2) 和 CO2 清除状态 (PetCO2) 有关的临床目标。
然后,通过各种选项微调 INTELLiVENT-ASV。例如,您可以决定是否想要手动设置 PEEP,还是想要 INTELLiVENT-ASV 在您定义的范围内设置 PEEP。审查或设置报警限值后,即准备好开始通气。
INTELLiVENT-ASV 在床旁实施您的策略。您无需频繁更改个人设置,仅在必要时监测并重新调整目标和策略。
INTELLiVENT-ASV 旨在使病人在您定义的目标范围内,并予以保持,同时维持肺保护通气 (
这些输入数据通过下面三个传感器测量:近端流量传感器提供肺力学指标和病人活动有关的数据,SpO2 和 CO2 传感器提供氧合状态和 CO2 清除状态有关的数据。
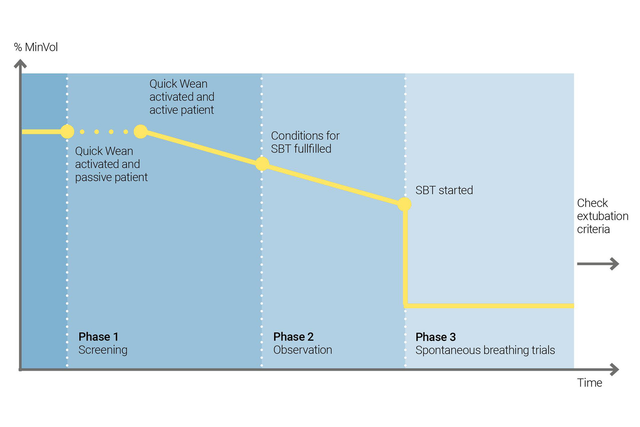
利用 INTELLiVENT-ASV 的快速撤机功能实施您的撤机方案。当病人自主呼吸时,您可以在通气过程中启用“快速撤机”。
您可以通过启用 SBT 配置“快速撤机”,以评估您的病人是否适于脱离呼吸机。您可以调整开始 SBT 的标准,运行 SBT 时使用的设置,以及中断 SBT 的标准。
INTELLiVENT-ASV 始终显示所有已执行 SBT 的历史。如果 SBT 失败,则 INTELLiVENT-ASV 恢复以前的通气设置。
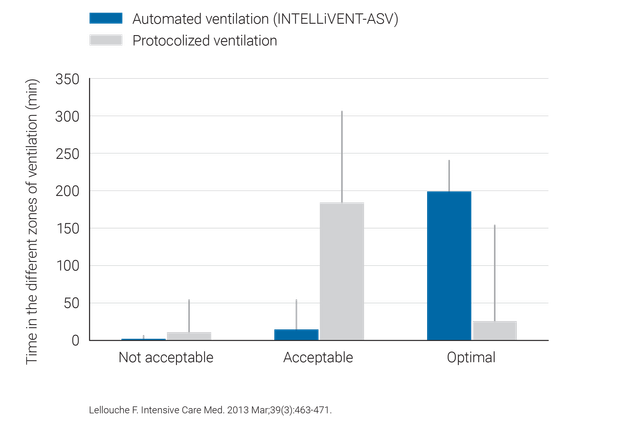
临床研究表明 INTELLiVENT-ASV 选择安全的驱动压力 (
相比常规通气,INTELLiVENT-ASV 无需大量手动调整,因此可减少医务人员的工作量(

INTELLiVENT-ASV 为 HAMILTON-C6、HAMILTON-G5, HAMILTON-C3、HAMILTON-C1 和 HAMILTON-T1 的选配模式,HAMILTON-S1 的标准模式。