
Author: Joe Hylton, MA, BSRT, RRT-ACCS/NPS, NRP, FAARC, FCCM, Clinical Applications Specialist, Hamilton Medical Inc.
Date of first publication: 15.07.2021
Waveform capnography is no stranger to intensive care/critical care medicine. It is a widely utilized airway management validation tool and is used extensively in the conscious sedation environment, as well as during interfacility transport of intubated patients requiring mechanical ventilation. Waveform capnography can provide timely, valuable information to a well-trained caregiver.

There are many factors that may affect the amount of carbon dioxide in the end-tidal gas (PetCO2). For the elimination of CO2, there is a close, continuous balance between the production of CO2 in the tissues, its transport in the blood, diffusion into the alveoli, and elimination by ventilation (
An increase or decrease in the patient's metabolic rate will lead to a change in CO2 production and, therefore, CO2 elimination as well. If both circulation and ventilation are stable - a state which can only be achieved in passive mechanically ventilated patients - CO2 monitoring can be used as an indicator of CO2 production. Fever, sepsis, pain, and seizures are all conditions that increase metabolism, causing a corresponding increase in CO2 production and, in turn, an increase in PetCO2. A decrease in metabolism occurs in patients who are hypothermic, or sedated and paralyzed. This lowers the production of CO2 and may lead to a decrease in PetCO2 if the minute ventilation does not increase at the same time (
The transport of CO2 to the lungs relies on proper cardiovascular function; therefore, any factor that alters cardiovascular function may also influence CO2 transport to the lungs (
The removal of CO2 from the lungs to the environment is affected by changes in respiratory function. Obstructive lung diseases, pneumonia, neuromuscular disorders, and central nervous system disorders that result in impaired respiratory function will therefore effect a change in PetCO2 value (
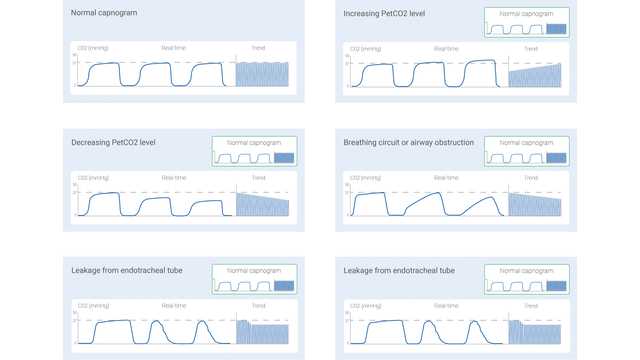
The measured CO2 signal can be recorded either as a function of time (time-based capnography) or expired volume (volumetric capnography). The amount of information potentially offered by these two different types of capnography varies significantly. Certain patterns in a time-based capnogram that are considered typical for specific clinical situations have been described in the literature. Some of the common ones are shown below in Figure 1.
However, time-based capnography also has limitations: It cannot provide an accurate estimate of the lung’s ventilation-perfusion status, nor can it be used to estimate the component of physiologic dead space. While not as simple and convenient as time-based capnography, volumetric capnography has the advantage of offering considerably more information.
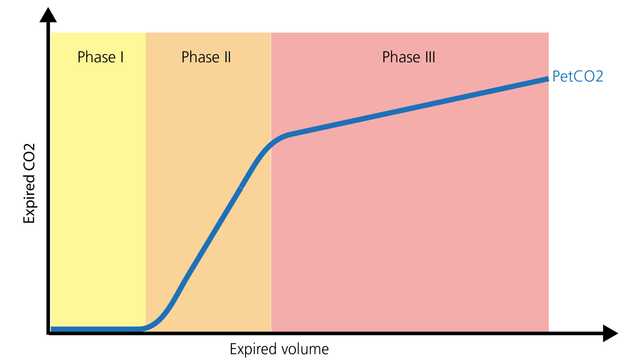
The normal shape of a volumetric capnogram consists of three phases. It is important to remember that the capnogram is representative of exhalation.
Capnography, whether time-based or volumetric, can provide valuable information to optimize monitoring and guide care in the patient requiring intrahospital/interhospital transport. It can be safely utilized with endotracheal tubes, tracheostomy tubes, and many supraglottic airways, provided an effective seal is present. Airway placement and patency, ventilation monitoring, and perfusion status are all areas where PetCO2 provides significant information. Another valuable parameter is the volume of carbon dioxide eliminated per minute (V'CO2), which allows caregivers to assess effective perfusion and volume resuscitative efforts (
In the intensive care unit, waveform capnography can continue monitoring of airway placement and patency, with various airway adjuncts. The dead space to tidal volume ratio (VD/Vt) is an important capnography measurement. An increasing VD/Vt ratio can represent a potential increase in mortality, based on the level of increase (
All Hamilton Medical ventilators provide volumetric capnography, either as a standard or optional feature (
Learn how to interpret a volumetric capnogram and get an overview of the benefits and clinical applications of volumetric capnography. Includes a self-test!

Learn everything you need to know in our guide to volumetric capnography: the volumetric capnogram, the capnography phases, what is dead space, the difference between anatomical dead space and alveolar dead space, PetCO during bronchospasm, V‘CO and CO2 elimination, and more.
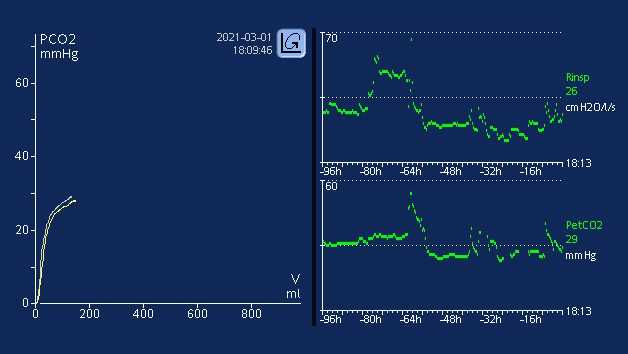
It is well established that metabolism, perfusion, and efficient lung function are paramount to effective CO2 transport and elimination (1). Changes in a patient’s metabolic state, perfusion, or lung function can affect CO2 elimination, sometimes drastically.