Author: Clinical Experts Group, Hamilton Medical
Date of first publication: 05.10.2020
Last change: 05.10.2020
(Originally published: 25.08.17) SW versions updatedHow do Hamilton Medical ventilators calculate V`alv and VDaw/VTE, and what are these parameters used for?

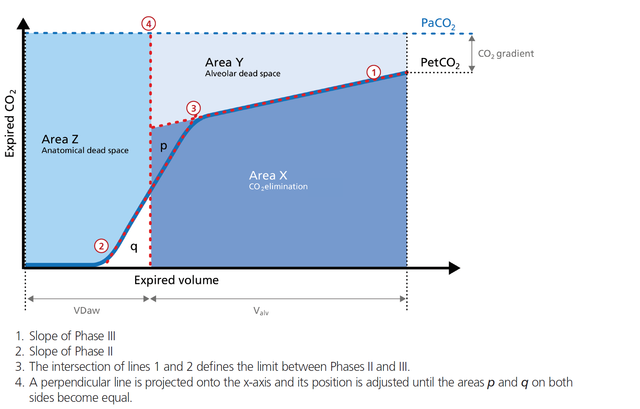
Alveolar minute ventilation in l/min: V'alv = RR * Vtalv = RR * (VTE - VDaw)
V'alv: Alveolar minute ventilation
RR: Respiratory rate
Vtalv: Alveolar tidal volume
VDaw: Anatomical dead space volume, calculated using the Vds-PIE slope method (
VTE: Expiratory tidal volume
In short, the alveolar minute ventilation (V`alv) reflects Area X in Figure 1 (below) multiplied by the respiratory rate.

Dead space fraction in %: VDaw/VTE = 100 x VDaw/VTE
VDaw: Anatomical dead space volume, calculated using the Vds-PIE slope method (
VTE: Expiratory tidal volume
The dead space fraction puts the expiratory tidal volume VTE in relation to the anatomical dead space volume VDaw.

Alveolar minute ventilation:
An increase in V‘alv can be seen after an effective recruitment maneuver and induces a transient increase in V‘CO2.
A decrease in V‘alv can indicate that fewer alveoli are participating in the gas exchange, for example, due to pulmonary edema.
The dead space fraction gives you an indication of how effective the ventilation is. A rising VDaw/Vte ratio may be an early sign of ARDS. In a normal lung, the VDaw/Vte ratio is between 25% and 30%. In patients with ARDS, a dead space fraction ≥ 60% was associated with higher mortality (
For more detailed information, please download our e-book on Volumetric Capnography below.
Relevant devices (
Learn how to interpret a volumetric capnogram and get an overview of the benefits and clinical applications of volumetric capnography. Includes a self-test!