Date of first publication: 06.12.2022

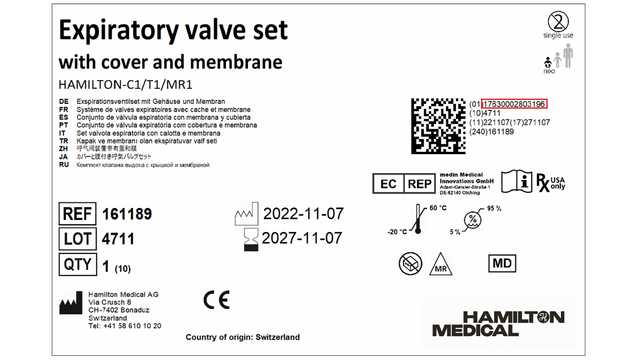
Pursuant to EU MDR 2017/745, manufacturers are obliged to assign a unique device identifier (UDI) to the different levels of packaging. To ensure compliance with this regulation, we have changed the primary and secondary packaging levels of our consumable lines.
Primary packaging level (layer of packaging closest to product):
Secondary packaging level (box):
Affected product lines are shown below in the table below.


| Product range | Part numbers |
| Breathing circuit sets for HAMILTON-H900 | 260161, 260186, 260185, 260187, 260200 |
| Pressure lines | 260174, 260176 |
| Flow sensors | 155500, 260177, 260179, 281637, 282049, 282051 |
| Breathing circuit sets | 260128, 260127, 260167, 260168, 260180, 260182, 260207, 260170, 260169, 260184,260240, 260241, 260244, 260087, 260094, 260145, 260144, 260257, 260206, 260239 |
| In2Flow Nasal cannulas | 10076606, 10076605, 10076604 |
| Expiratory valve sets | 160176, 161186, 161189, 950158 |