Grâce à notre mode de ventilation intelligente, vous passez du rôle de manipulateur de boutons à celui de superviseur. L'INTELLiVENT-ASV réduit le nombre d'interventions manuelles sur le ventilateur (
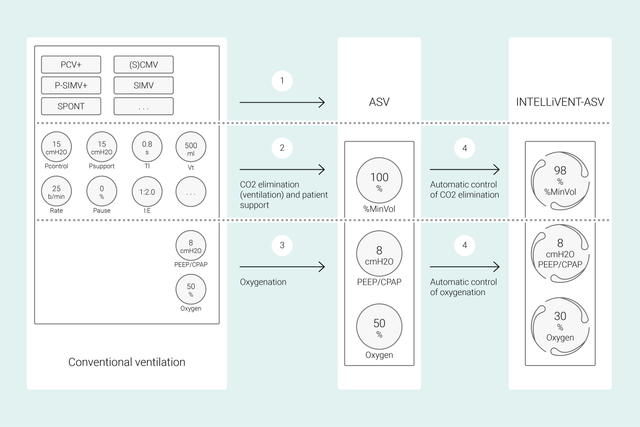
Avec les modes classiques, vous définissez plusieurs réglages sur le ventilateur tels que le volume courant ou la pression, la fréquence respiratoire, la FiO2, la PEP et le temps expiratoire et inspiratoire pour atteindre des objectifs cliniques. En outre, tous ces réglages doivent être réévaluées et réajustés fréquemment.
Grâce à l'INTELLiVENT-ASV, les cibles et stratégies cliniques que vous définissez pour l'oxygénation et la ventilation sont au cœur du processus. Une fois que vous avez défini ces cibles, vous pouvez décider dans quelle mesure l'INTELLiVENT-ASV doit contrôler l'oxygénation et la ventilation pour les atteindre.
L'INTELLiVENT-ASV sélectionne ensuite automatiquement des réglages de ventilateur, gère la transition entre les états passifs et actifs et soutient activement vos protocoles de sevrage grâce à l'option Sevrage rapide.
De nombreuses études internationales ont montré la sécurité et les performances de l'INTELLiVENT-ASV dans différents scénarios cliniques - depuis les patients ayant subi une chirurgie cardiaque (


Dans cette vidéo, le Dr Jean-Michel Arnal, responsable des soins intensifs, fait une présentation rapide des principaux réglages et fonctionnalités du mode INTELLiVENT-ASV sur un patient réel, admis en soins intensifs.
Pour commencer, vous devez définir la taille, le sexe du patient et, si nécessaire, un critère spécifique : SDRA, Hypercp. chr. ou Lésion céréb. Ensuite, vous définissez les cibles cliniques en matière d'oxygénation (SpO2) et d'élimination de CO2 (PetCO2) pour votre patient.
Vous avez alors plusieurs options pour régler avec précision l'INTELLiVENT-ASV. Par exemple, vous pouvez choisir de définir la PEP manuellement ou décider que l'INTELLiVENT-ASV définisse la PEP dans une plage que vous aurez définie. Une fois les limites d'alarme vérifiées ou définies, vous êtes prêt à commencer la ventilation.
L'INTELLiVENT-ASV met en œuvre votre stratégie au chevet du patient. Plutôt que de modifier sans cesse les réglages individuels, vous surveillez et réajustez les cibles et la stratégie si nécessaire.
L'INTELLiVENT-ASV amène le patient dans la plage cible que vous avez définie et l'y maintient, tout en garantissant une ventilation de protection pulmonaire (
Ces paramètres sont mesurés par trois capteurs : le capteur de débit proximal fournit des données sur la mécanique pulmonaire et l'activité respiratoire du patient, alors que les capteurs de SpO2 et de CO2 fournissent des données sur l'oxygénation et l'élimination de CO2.
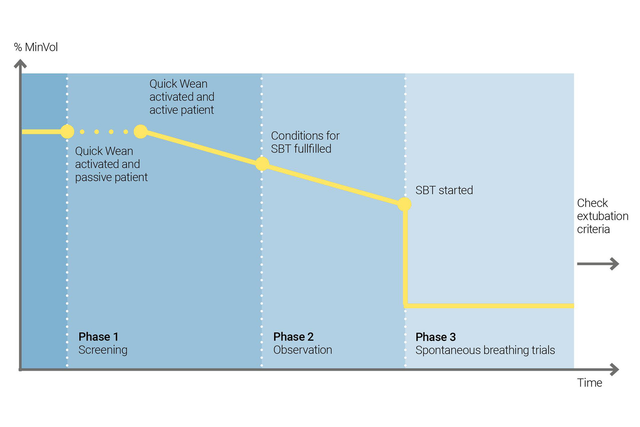
Utilisez la fonction Sevrage rapide de l'INTELLiVENT-ASV pour mettre en œuvre votre protocole de sevrage. Vous pouvez activer la fonction Sevrage rapide pendant la ventilation lorsque le patient respire spontanément.
Vous pouvez configurer la fonction Sevrage rapide en activant les EVS pour évaluer la capacité du patient à être séparé du ventilateur. Vous ajustez les critères pour commencer une EVS, les réglages à utiliser pendant l'exécution de l'EVS et les critères pour y mettre fin.
L'INTELLiVENT-ASV affiche toujours l'historique de toutes les EVS effectuées. En cas d'échec d'une EVS, l'INTELLiVENT-ASV restaure les réglages de ventilation précédents.
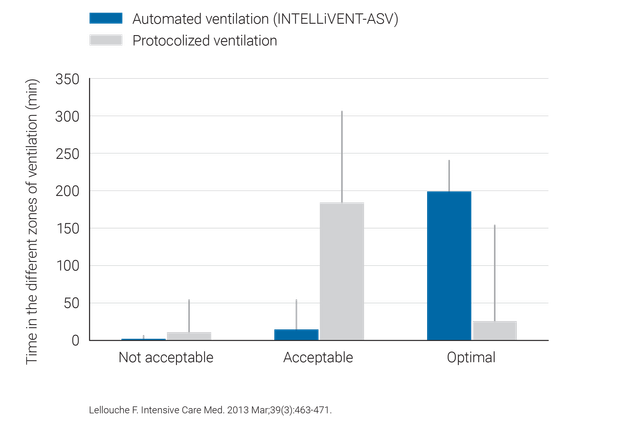
Des études cliniques ont démontré que l'INTELLiVENT-ASV sélectionne une pression motrice sûre (
L'INTELLiVENT-ASV nécessite moins de réglages manuels que dans la ventilation classique, ce qui réduit la charge de travail du personnel soignant (

L'INTELLiVENT-ASV est disponible en option sur les ventilateurs HAMILTON-G5, HAMILTON-C6, HAMILTON-C3, HAMILTON-C1 et HAMILTON-T1, et en mode standard sur le HAMILTON-S1.