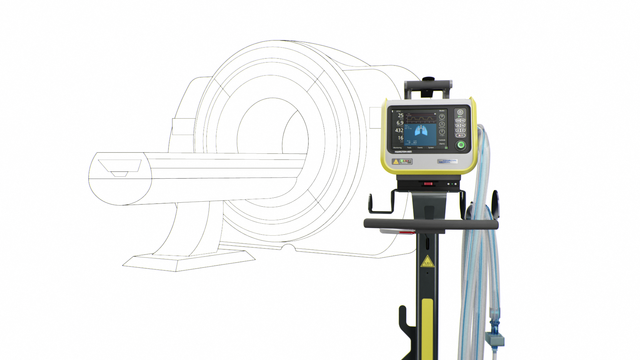
我们的 MRI 专家。在 MRI 检查期间提供高端通气

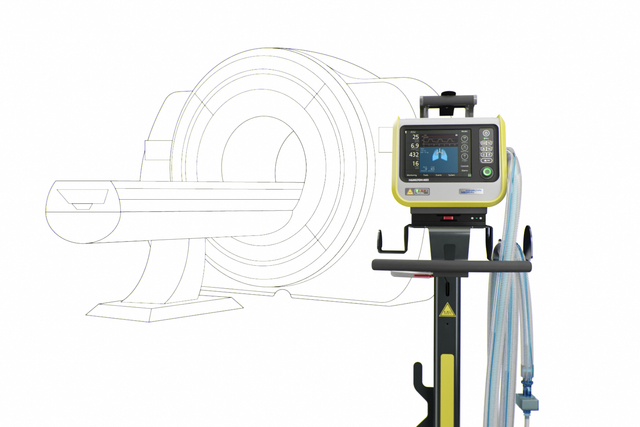



TeslaSpy 帮助您使呼吸机与 MRI 扫描仪保持安全距离。

在适合 MRI 科室的各种不同长度呼吸管路之间选择。

台车上的自锁式刹车自动锁定到位,预防呼吸机意外滚向 MRI 扫描仪。

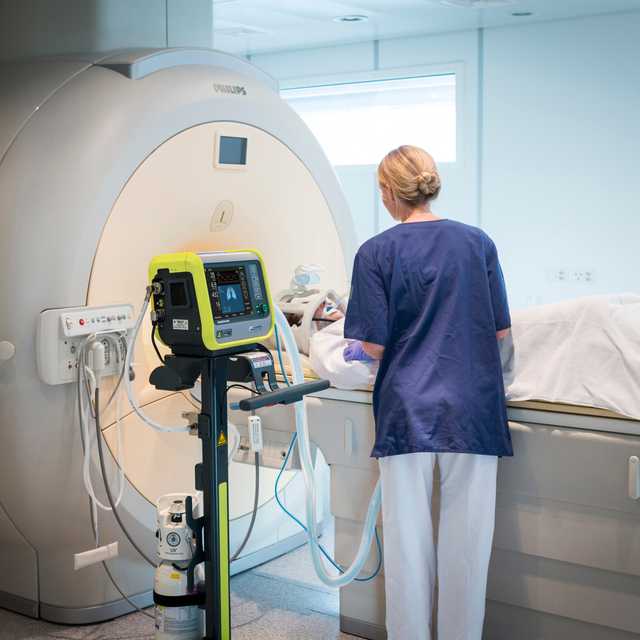
HAMILTON-MR1 允许您靠近 MRI 扫描仪,这是因为它经过专门设计,可承受高达 50 mT 的静态磁场。相比磁场屏蔽较弱的呼吸机,放置设备时它为您提供更多自由,更大移动范围,以及更高工作流程和病人设置的灵活性。
更靠近扫描仪同时也意味着您可以使用更短的呼吸管路。优点是减少可压缩容积,从而有利于提高整个通气质量。

转运套件上的快速释放按钮允许您通过按下按钮,断开设备与台车。重新连接也这么简单。
从各个角度发现 HAMILTON-MR1,点击热点,以了解更多信息。
| 病人组 | 成人/儿童、新生儿 |
|---|---|
| 外形尺寸(宽x深x高) | 320 x 220 x 270 mm(呼吸机主机) 630 x 630 x 1400 mm(含台车) |
| 重量 | 6.8 kg(15 磅) 21 kg(46.2 磅)(含台车) |
| 监视器尺寸和分辨率 | 214 mm(8.4 英寸)对角线 640 x 480 像素 |
| 可拆卸式监视器 | |
| 电池运行时间 | 两块电池 8 小时 |
| 热插拔电池 | |
| 气源 | 集成涡轮 |
| O2 接头 | DISS 标准接口(CGA 1240 认证)或 NIST 通用接口 |
| 连接 | USB 端口 |
| 音量 | 43 dB(在正常运行情况下) |
| 容量控制、流量控制 | |
|---|---|
| 定量、适应性压力控制 | |
| Pressure-controlled | |
| 智能通气 | ASV®、INTELLiVENT‑ASV®(可选模式) |
| 无创通气 | |
| 高流量 | |
| 肺力学指标可视化(动态肺) | |
|---|---|
| 病人呼吸机依赖性可视化 | |
| 二氧化碳图 | |
| 氧饱和度监测 | |
| Lung stress and strain monitoring (Lung Impact panel) | |
| 食道压测量 |
| 肺复张性评估和肺复张 (P/V Tool Pro) | |
|---|---|
| 人机同步 (IntelliSync+) | |
| CPR 通气 | |
| Hamilton Connect 模块 | |
| Suctioning tool | |
| SpeakValve compatibility | |
| On-screen help | |
| O2 assist |
| 远程连接至 HAMILTON-H900 湿化器 | |
|---|---|
| 集成 IntelliCuff 气囊压力控制器 | |
| 集成气动雾化器 | |
| 集成 Aerogen 雾化器 | |
| 与 Sedaconda ACD-S 麻醉剂输送系统的兼容性 |
根据病人的肺力学指标和呼吸用力,ASV 通气模式每天 24 时从插管到拔管连续调整每次呼吸时的呼吸频率、潮气量和吸气时间。
无创通气模式提供压力支持流速切换的自主呼吸(NIV 和 NIV-ST 模式)和压力控制时间切换的指令呼吸 (NIV-ST)。
与使用压缩空气的呼吸机相比,我们的涡轮驱动呼吸机能够提供更高的峰值流量。这就保证了即便漏气严重也具有最佳性能。
高流量鼻导管治疗(
我们的独立高流量氧疗设备 HAMILTON-HF90 同样提供了此疗法 (
IntelliSync+ 通过每秒数百次波形持续分析波形,能够立即检测病人用力和循环,并实时启动吸气和呼气。
IntelliSync+ 适用于有创和无创通气,无论采用哪种通气模式。
动态肺面板向您显示下列重要监测数据的实时图表视图:
说话瓣膜选项让气管切开的病人说话,而且允许他们甚至在接受通气治疗时吞咽。
调节呼吸机的监测、触发和报警管理,以在压力控制模式 (PCV+, SPONT, PSIMV+) 下与说话瓣膜兼容。
在 nCPAP 模式下,病人通过持续气道正压通气获得支持。在我们的流量控制型设备中,目标 CPAP 值通过调节呼吸气体流量进行设定。为补偿可能发生的漏气(例如经口或鼻腔漏气),可启用 LeakAssist 功能。该能够可通过补充呼吸气体流量维持预设的气道压力。
集成气动雾化器完全与吸气和呼气时间同步。
集成同步 Aerogen 雾化系统作为一个选配件提供 (
输送药物气溶胶粒子的细水雾有助于您恢复支气管痉挛、提高通气效率和减少高碳酸血症 (

我们预组装的呼吸装置包括操作呼吸机所需的基本耗材,而且方便地放在一个包装袋中。
我们的所有基本耗材都专门为保证制造商质量的 Hamilton Medical 哈美顿医疗公司呼吸机开发。

为管理通气,您通常需要设置多个参数,例如,压力、容量、吸气和呼气触发、气囊压力等。每次您的病人状况改变时,您需要进行一次或多次调节。
为简化此过程和减少人工操作,我们创建了一系列解决方案:
适应性支持通气 (ASV) 是一种根据病人的肺力学指标和呼吸用力连续适应呼吸频率、潮气量和吸气时间的通气模式。研究表明,ASV 可缩短各种病人群体的机械通气时间,而且手动设置更少 (
气囊压力管理的常规解决方案需要您手动监测和调节气囊压力。
IntelliCuff 通过连续测量和自动维持所设置的成人、儿童和新生儿病人的气囊压力,安全管理病人的气道 (

无论何时出现问题,呼吸机都会利用报警灯、声音和信息栏警示您。
屏幕上的帮助内容向您提供有关如何解决报警的建议。

我们希望我们的呼吸机尽可能快速与病人脱离。因此我们向您提供工具帮助您实施您的撤机方案。
其中包括旨在鼓励自主呼吸的可视帮助和通气模式。

我们不断努力进一步革新我们的产品。添加新的功能和改善现有功能,以确保您在您的呼吸机寿命期间始终拥有最新的通气技术。


无论用于 ICU、MRI 科室或病人转运,所有 Hamilton Medical 哈美顿医疗公司呼吸机的用户界面操作方式均相同。
我们的通气酷屏将复杂的数据集成到直观的可视化图像。
我们围绕最高病人安全性和易用性开发我们的附件。我们尽可能将附件集成到我们的呼吸机,以简化整个呼吸机系统的操作。

我们的通气极客团队很乐意帮助您选择最适合您临床护理环境的通气设备,并帮助您实现治疗目标。获取个性化报价或安排电话回访,了解更多信息。