
Author: Karjaghli Munir, Respiratory Therapist, Hamilton Medical Clinical Application Specialist
Date of first publication: 01.03.2024
Learn everything you need to know in our guide to NIV ventilation: from the medical abbreviation, to what noninvasive ventilation is, when to use it, selecting the interface and settings, and more.

Noninvasive ventilation is commonly referred to as NIV.
The term noninvasive positive-pressure ventilation (abbreviated NPPV or NIPPV) was previously used to distinguish it from noninvasive negative-pressure ventilation, but given the latter's rarity nowadays, the simpler term NIV is more convenient. As there is now a range of ventilators available for NIV, use of the product name BIPAP (Bilevel positive airway pressure) as a generic term for NIV should be avoided.
Other abbreviations are explained directly in the text.
Noninvasive positive-pressure ventilation involves the delivery of oxygen into the lungs via positive pressure without the need for endotracheal intubation. It is used in both acute and chronic respiratory failure, but requires careful monitoring and titration to ensure its success and avoid complications (
Over the past century, noninvasive ventilation (NIV) has improved dramatically and been used to treat respiratory failure from multiple etiologies. It has been proven more effective in preventing intubation compared to standard oxygen therapy in the acute setting (
Respiratory support may be delivered using continuous positive airway pressure (CPAP) devices or those that deliver bilevel positive airway pressure (pressure-support ventilation, PSV). For the purposes of this article, the name NIV covers both CPAP and PSV (

We can divide the goals and benefits of NIV into the acute and long-term care setting as follows.
Benefits of NIV in acute care settings (
Benefits of NIV in long-term care setting (
Noninvasive ventilation works by creating positive airway pressure, i.e., the pressure outside the lungs is greater than the pressure inside the lungs. This causes air to be forced into the lungs (down the pressure gradient), lessening the respiratory effort and reducing the work of breathing.
It also helps to keep the chest and lungs expanded by increasing the functional residual capacity (the amount of air remaining in the lungs after expiration) after normal (tidal) expiration; this is the air in the alveoli available for gas exchange (
The following modes are noninvasive:
CPAP aims to deliver one continuous level of positive pressure throughout both the inspiratory and expiratory phases of breathing.
It improves oxygenation by opening collapsed airways, improving functional residual capacity (FRC), and improving preload and afterload in cardiogenic pulmonary edema (
CPAP improves lung compliance and therefore reduces the effort required for breathing by preventing alveolar collapse and counteracting the excessive intrinsic PEEP seen in obstructive lung conditions such as COPD (
Good to know: When ΔPsupport /∆Pinsp is set to zero in NIV and NIV-ST, the ventilator functions like a conventional CPAP system.
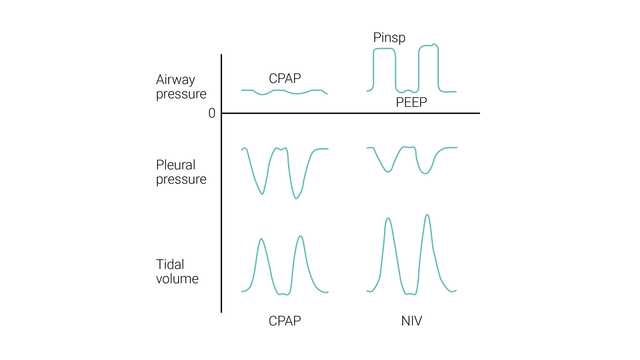
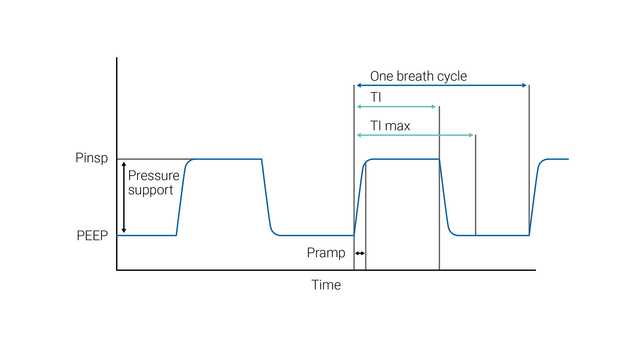
NIV (PSV) aims to deliver two levels of positive airway pressure support. The lower level is similar to CPAP; however, it is more commonly called positive end-expiratory airway pressure (PEEP) as it is present only at the expiratory phase of breathing.
The patient’s inspiratory effort is assisted by the ventilator at a preset level of inspiratory pressure (ΔPsupport). Inspiration is triggered and cycled by the patient's effort. During NIV, the patient determines the respiratory rate, inspiratory time, and tidal volume.
The size of the breath (tidal volume) generated in a particular patient is dependent on the ΔPsupport setting – the higher the ΔPsupport setting, the greater the tidal volume.
ETS (expiratory trigger sensitivity) determines the spontaneous inspiratory time by cycling to expiration once the inspiratory flow decreases to a preadjusted percentage of the peak inspiratory flow.
In case the ETS criteria are not met (leakage), the inspiratory time can also be limited by TI max (maximum inspiratory time).
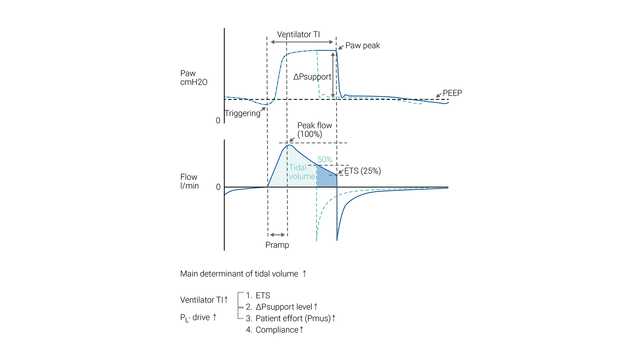
In S/T mode, the clinician sets the inspiratory pressure (∆Pinsp) and expiratory pressure (PEEP), respiratory rate, and inspiratory time. The patient may initiate breaths that are supported to the ∆Pinsp level, as in the NIV mode, but if the patient fails to make an inspiratory effort within a set interval (that is defined by the set respiratory rate), the machine triggers inspiration to the set ∆Pinsp level. ∆Pinsp then cycles to PEEP based on the inspiratory time period.
NIV-ST with the backup rate is useful in the case of apnea or periodic breathing (
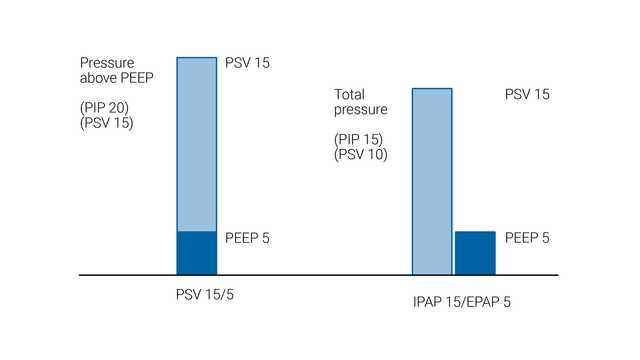
The NIV and NIV-ST modes are types of noninvasive positive pressure ventilation. Pressure support is generally considered to be the same as CPAP/BIPAP, except that pressure support is delivered by a ventilator and BIPAP through a noninvasive ventilator.
In NIV and NIV-ST, the level of pressure support is applied as pressure above baseline PEEP. However, the approach is different with bilevel ventilators where IPAP (inspiratory positive airway pressure) and EPAP (expiratory positive airway pressure) are set. In this configuration, the difference between IPAP and EPAP is the level of pressure support.
In order to minimize the risk of failure or complications, every patient should be properly assessed for suitability to receive NIV safely.
Indications for NIV use include patients who have:
Patients should be closely monitored during the first 24 hours after initiating NIV, as this is the period with the highest rate of treatment failure. Although data points at presentation such as a high RR (respiratory rate), low arterial pH values or low PaO2/FiO2 can help predict failure, the most robust predictor of treatment failure during this period is failing to show an improvement in these parameters at 1–2 h after initiating NIV treatment (
| Clinical indication | Certainty of evidence | Recommendation |
| Hypercapnia with COPD exacerbation | High | Strong recommendation for |
| Cardiogenic pulmonary edema (CPE) | Moderate | Strong recommendation for |
| Immunocompromised | Moderate | Conditional recommendation for |
| Post-operative patients | Moderate | Conditional recommendation for |
| Palliative care | Moderate | Conditional recommendation for |
| Trauma | Moderate | Conditional recommendation for |
| Weaning in hypercapnic patients | Moderate | Conditional recommendation for |
| Post-extubation respiratory failure | Low | Conditional recommendation against |
| Obesity hypoventilation syndrome (OHS) | Low | Conditional recommendation for |
| Neuromuscular disease and chest wall disease | Low | Conditional recommendation for |
| Prevention of hypercapnia in COPD exacerbation | Low | Conditional recommendation against |
| Post-extubation in high-risk patients (prophylaxis) | Low | Conditional recommendation for |
| De novo respiratory failure | No certain evidence | No recommendation made |
| Acute asthma exacerbation | No certain evidence | No recommendation made |
Absolute:
Relative:
(
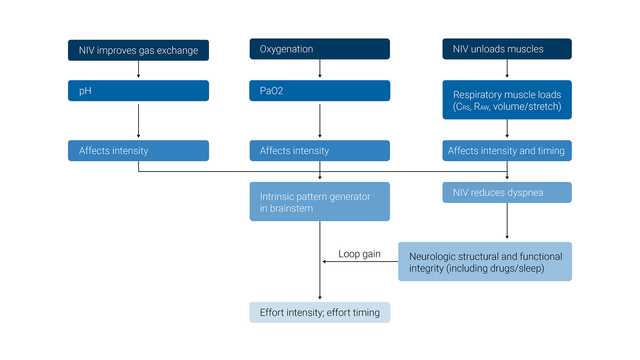
The primary desired effect of NIV is to maintain adequate levels of PO2 and PCO2 in the arterial blood while also unloading the inspiratory muscles.
The physiologic effects of noninvasive ventilation are the following:
(
(
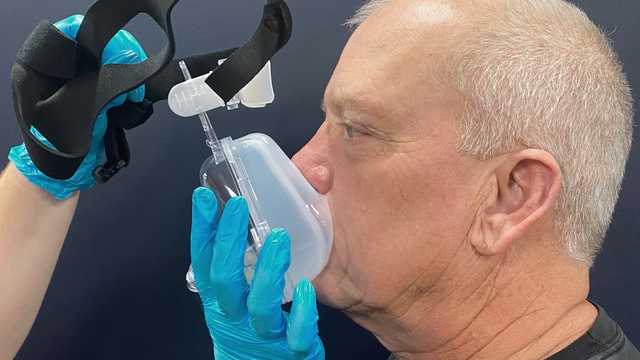
Select the mask designed for use with a critical care ventilator (without entrainment valves or leak port).
The wrong size of mask, incorrect positioning, or an insufficient mask seal may all lead to the patient discomfort and pressure injuries. It is important to choose an interface that is the correct size for the individual patient, evaluate the mask fit and placement on the face, and reposition the mask as needed to minimize leaks.
Good to know: NIV interfaces with entrainment valves are designed for noninvasive ventilators that use a single-limb circuit. The entrainment valve is required to prevent asphyxia if the ventilator fails or the tubing becomes disconnected. Masks with leak ports should only be used with single-limb circuit ventilators and should not be used with Hamilton Medical ventilators.
(

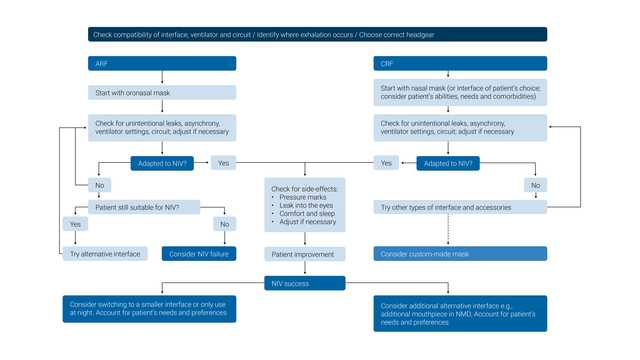

Recommendations (
Dyspnea can cause feelings of anxiety and fear. For this reason, the healthcare professional or the patient should hold the mask in place when applying it for the first time.
This way the mask can be removed quickly if the patient begins to panic or needs to communicate. A strategy of starting with low pressures can help patients adjust to NIV more readily.

(

Emergency alarms are important for recognizing a deterioration in the patient's condition and alert staff in the following situations:
(
Failure of NIV has usually been defined as a need for intubation due to a lack of improvement in arterial blood gases and clinical parameters, or death (
Success predictors:
Failure predictors:
In a large prospective cohort study, the HACOR scale predicted NIV failure after 1 h of treatment with high specificity (90%) and good sensitivity (72%) (
Patient-ventilator synchronization is an important issue that can influence the efficacy and success of NIV.
The most common phenomenon is ineffective triggering (patient effort is not recognized by the ventilator; may be secondary to high AutoPEEP or inappropriate inspiratory trigger sensitivity), followed by auto-triggering (delivery of preset pressure in the absence of patient effort) and double triggering (consecutive delivery of two preset pressure support events within an interval of less than half the mean inspiratory time due to the patient’s continued effort) (
Synchrony between the patient and ventilator should be checked frequently. Asynchronies can be detected by observing the patient and asking them simple questions. The most practical method should be analysis of the pressure and flow waveforms (
Hamilton Medical ventilators offer a functionality for leak compensation, IntelliTrig, during the full breath cycle to increase patient-ventilator synchrony and reduce the risk of auto-triggering. Using IntelliTrig, the ventilator identifies the leak by measuring the flow at the airway opening, and uses this data to automatically adjust the gas delivery while remaining responsive to the set inspiratory and expiratory trigger sensitivity.
Hamilton Medical ventilators also have the optional feature, IntelliSync+, which continuously analyzes waveform shapes and is able to detect patient efforts immediately, then initiate inspiration or expiration in real-time (
Together with our HAMILTON-C1, the compact solution for noninvasive ventilation, all Hamilton Medical ventilators offer the NIV option (it is available on the HAMILTON‑C6, the HAMILTON‑C3, and on the HAMILTON‑C1/T1/MR1).

Get an overview of benefits and clinical relevance of noninvasive ventilation, as well as practical information about choosing the right interface, adjusting the settings, and monitoring your patients.