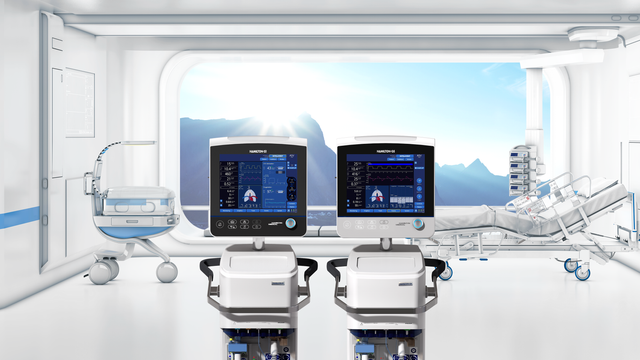
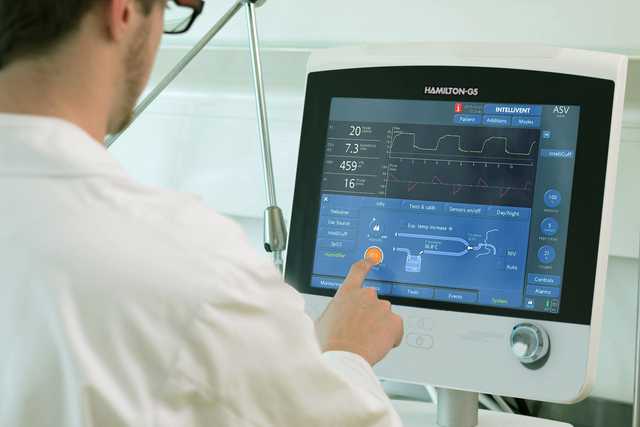
在重症监护室,HAMILTON-G5 和 HAMILTON-S1 呼吸机是所有病人群体的忠实支持者,从新生儿到成人。凭借其众多高端功能,他们可以支持您的病人,满足他们所有的通气需求,从高流量氧疗到有创通气。在适当时候,INTELLiVENT-ASV 等高级模式甚至可以帮助您撤机 (

有了 HAMILTON-G5 上广泛的可选功能,您可以创建自己的定制解决方案,向您的病人提供个性化肺保护通气:
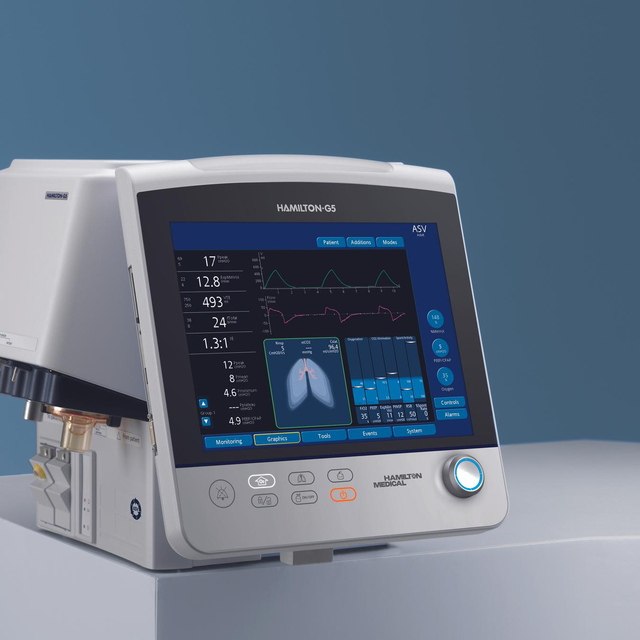
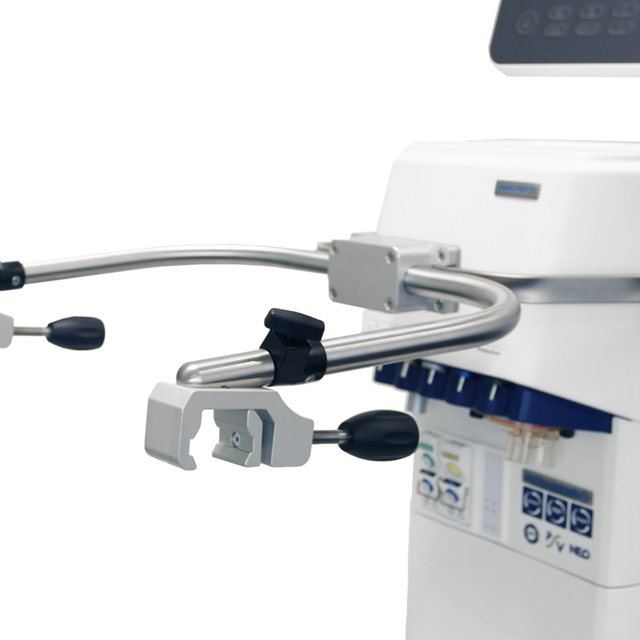


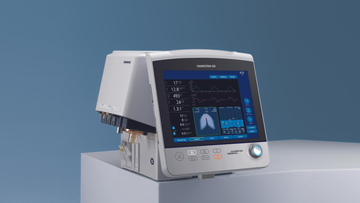

这款呼吸机安装非常灵活!您可以将其装在台车上,连接到悬挂系统上,拆下其显示器或将其安装在台架上。
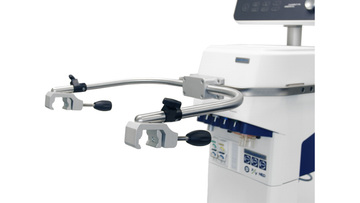
通过独特的呼吸机连接选项可以直接从呼吸机的显示屏上操作 HAMILTON-H900 湿化器。您可以访问所有控件、监测参数和报警,并根据需要予以调节。
湿化器也可以根据所选的通气模式自动选择湿化模式(有创、无创或高流量)。
从各个角度发现 HAMILTON-G5/S1,点击热点,以了解更多信息。
| 病人组 | 成人/儿童、新生儿 |
|---|---|
| 外形尺寸(宽x深x高) | 500 x 450 x 440 mm(呼吸机主机) 580 x 600 x 1300 mm(安装在轨道上的最小监视器) 580 x 600 x 1500 mm(安装在轨道上的最大监视器) |
| 重量 | 呼吸机主机、监视器和固定架:38 kg(83.8 磅) 57 kg(125.6 磅)(含标准台车、监视器和呼吸机主机) |
| 监视器尺寸和分辨率 | 381 mm(15 英寸)对角线 1024 x 768 像素 |
| 可拆卸式监视器 | |
| 电池运行时间 | 一块电池 1 小时 |
| 热插拔电池 | |
| 气源 | 需要压缩空气 |
| O2 接头 | DISS (CGA 1240) 或 NIST(可选)、NF(可选) |
| 连接 | CompactFlash、USB、DVI、COM (RS-232)、专用接口 |
| 音量 | 38.6 dB(在正常运行情况下) |
| 容量控制、流量控制 | |
|---|---|
| 定量、适应性压力控制 | |
| Pressure-controlled | |
| 智能通气 | ASV®、INTELLiVENT®-ASV®(HAMILTON-G5 选配功能,HAMILTON-S1 标准功能) |
| 无创通气 | |
| 高流量 | |
| 肺力学指标可视化(动态肺) | |
|---|---|
| 病人呼吸机依赖性可视化 | |
| 二氧化碳图 | |
| 氧饱和度监测 | |
| Lung stress and strain monitoring (Lung Impact panel) | |
| 食道压测量 |
| 肺复张性评估和肺复张 (P/V Tool Pro) | |
|---|---|
| 人机同步 (IntelliSync+) | |
| CPR 通气 | |
| Hamilton Connect 模块 | |
| Suctioning tool | |
| SpeakValve compatibility | |
| On-screen help | |
| O2 assist |
| 远程连接至 HAMILTON-H900 湿化器 | |
|---|---|
| 集成 IntelliCuff 气囊压力控制器 | |
| 集成气动雾化器 | |
| 集成 Aerogen 雾化器 | |
| 与 Sedaconda ACD-S 麻醉剂输送系统的兼容性 |
根据病人的肺力学指标和呼吸用力,ASV 通气模式每天 24 时从插管到拔管连续调整每次呼吸时的呼吸频率、潮气量和吸气时间。
INTELLiVENT-ASV 智能通气模式持续调整病人的通气和氧合状态。
它根据临床医生设定的目标值和病人的生理输入设置分钟通气量、PEEP 和氧浓度。
IntelliSync+ 通过每秒数百次波形持续分析波形,能够立即检测病人用力和循环,并实时启动吸气和呼气。
IntelliSync+ 适用于有创和无创通气,无论采用哪种通气模式。
您可以使用 P/V Tool 评估肺复张性和确定肺复张策略。
此外,您也可将其用于进行持续充气肺复张操作和测量肺容量的增加。
跨肺压监测可优化 PEEP、潮气量和吸气压力(
将其与 P/V Tool 配合使用可评估肺复张性和执行肺复张术。
通过独特的呼吸机连接选项可以直接从呼吸机的显示屏上操作 HAMILTON-H900 湿化器(
湿化器也可以根据所选的通气模式自动选择湿化模式(有创、无创或高流量)。
集成气动雾化器完全与吸气和呼气时间同步。
集成同步 Aerogen 雾化系统作为一个选配件提供 (
输送药物气溶胶粒子的细水雾有助于您恢复支气管痉挛、提高通气效率和减少高碳酸血症 (
IntelliCuff 可实时对用户设置的气管内插管或气管切开插管的气囊压力进行持续测量和自动维持 (
高流量鼻导管治疗(
我们的独立高流量氧疗设备 HAMILTON-HF90 同样提供了此疗法 (
近端流速和二氧化碳测量使我们的呼吸机能生成最新的容积二氧化碳图,为评估通气质量和新陈代谢活动提供生要依据。
通气状态面板显示与病人的呼吸机依赖性相关的六个参数,包括氧合状态、CO2 清除状态和病人活动。
各栏中上下移动的浮动指示器显示给定参数的当前值。
快速撤机是 INTELLiVENT-ASV 模式的一个功能,其可提供对病人状况的持续动态监测和控制,从而评估病人是否适于拔管。
自动自主呼吸试验 (SBT) 是 INTELLiVENT-ASV 模式中快速撤机功能的一部分,并为您提供执行全控型 SBT 的选项。
动态肺面板向您显示下列重要监测数据的实时图表视图:
呼吸机可根据所选的监测参数组合显示动态环图。有了趋势图功能,您可以看到针对您选择的监测参数和时间框所显示的趋势数据。
设备持续将监测参数保存在其存储器中,即使在待机时也不停止。
氧饱和度选项提供集成无创氧饱和度测量,数据方便地显示在您的呼吸机上。
我们还提供氧饱和度传感器的全面组合方案。
无创通气模式提供压力支持流速切换的自主呼吸(NIV 和 NIV-ST 模式)和压力控制时间切换的指令呼吸 (NIV-ST)。
与使用压缩空气的呼吸机相比,我们的涡轮驱动呼吸机能够提供更高的峰值流量。这就保证了即便漏气严重也具有最佳性能。
在 nCPAP 模式下,病人通过持续气道正压通气获得支持。在我们的流量控制型设备中,目标 CPAP 值通过调节呼吸气体流量进行设定。为补偿可能发生的漏气(例如经口或鼻腔漏气),可启用 LeakAssist 功能。该能够可通过补充呼吸气体流量维持预设的气道压力。

我们预组装的呼吸装置包括操作呼吸机所需的基本耗材,而且方便地放在一个包装袋中。
我们的所有基本耗材都专门为保证制造商质量的 Hamilton Medical 哈美顿医疗公司呼吸机开发。

为管理通气,您通常需要设置多个参数,例如,压力、容量、吸气和呼气触发、气囊压力等。每次您的病人状况改变时,您需要进行一次或多次调节。
为简化此过程和减少人工操作,我们创建了一系列解决方案:
适应性支持通气 (ASV) 是一种根据病人的肺力学指标和呼吸用力连续适应呼吸频率、潮气量和吸气时间的通气模式。研究表明,ASV 可缩短各种人群的机械通气时间,而且手动设置更少 (
我们的智能通气模式 INTELLiVENT-ASV 使您从操作者转变为监督者,减少与呼吸机手动互动的次数 (
IntelliSync+ 至少每秒一百次连续分析波形信号。这使 IntelliSync+ 可以立即检测病人用力情况,并实时启动吸气和呼气,因此替代传统的吸气和呼气触发设置。
气囊压力管理的常规解决方案需要您手动监测和调节气囊压力。
IntelliCuff 通过连续测量和自动维持所设置的成人、儿童和新生儿病人的气囊压力,安全管理病人的气道 (

我们希望我们的呼吸机尽可能快速与病人脱离。因此我们向您提供工具帮助您实施您的撤机方案。
其中包括旨在鼓励自主呼吸的可视帮助和通气模式。
我们的在线学院提供易于遵循的学习路径,以使您尽快熟悉 Hamilton Medical 哈美顿医疗公司产品和技术。

HAMILTON-S1 配备所有选配功能,以及 INTELLiVENT-ASV。

我们不断努力进一步革新我们的产品。添加新的功能和改善现有功能,以确保您在您的呼吸机寿命期间始终拥有最新的通气技术。


无论用于 ICU、MRI 科室或病人转运,所有 Hamilton Medical 哈美顿医疗公司呼吸机的用户界面操作方式均相同。
我们的通气酷屏将复杂的数据集成到直观的可视化图像。
我们围绕最高病人安全性和易用性开发我们的附件。我们尽可能将附件集成到我们的呼吸机,以简化整个呼吸机系统的操作。