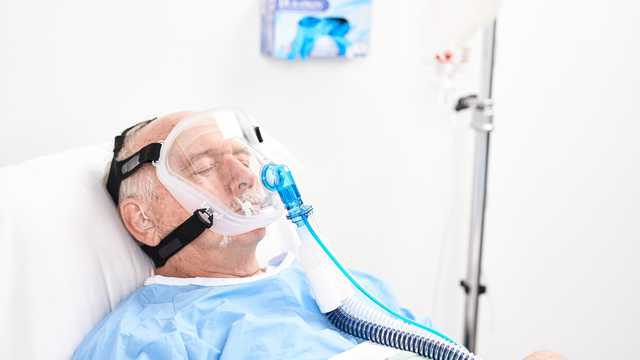
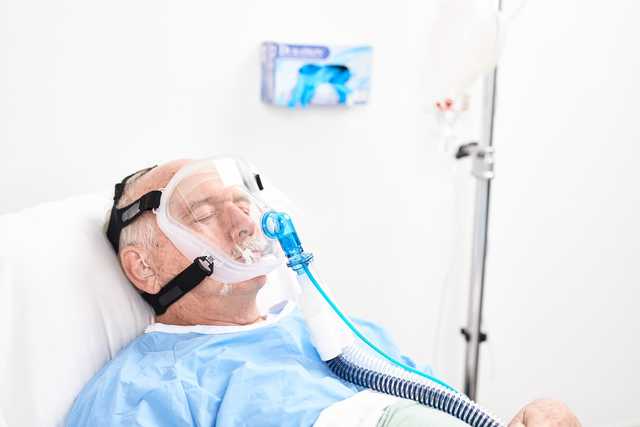
Le maschere facciali, oronasali, e nasali per NIV sono maschere riutilizzabili monopaziente compatibili con la ventilazione non invasiva dei pazienti che respirano spontaneamente (modalità NIV e NIV-ST).

Il cuscinetto con doppio profilo sporgente consente di posizionare la maschera sul viso del paziente e creare una tenuta efficace senza dover premere.
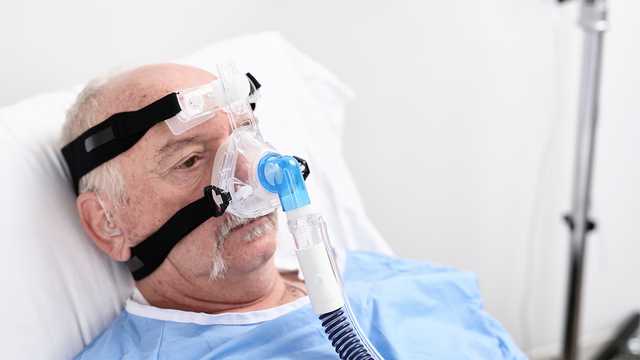
OmniClip è un componente regolabile presente nella zona frontale imbottita di tutte le maschere oronasali e nasali per NIV Hamilton Medical. È possibile regolare la maschera verso l'interno, l'esterno, l'alto o il basso per ottenere una migliore adesione al viso del paziente e ridurre la pressione sul dorso del naso.
Con OmniClip, è anche possibile spostare la maschera verso l'alto e scoprire il viso del paziente per somministrare acqua, cibo o medicinali.
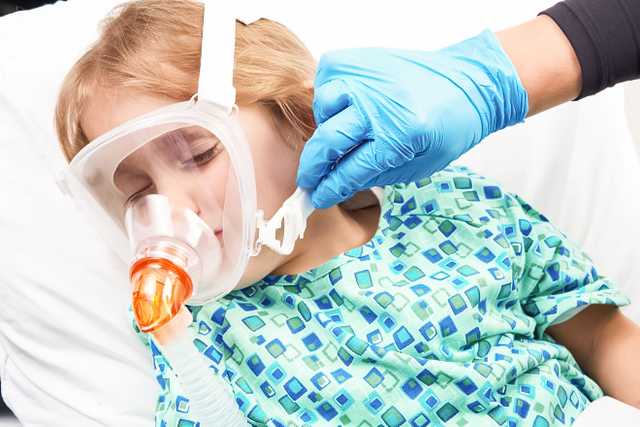
La fascia per la testa è dotata di fascette regolabili con clip di facile utilizzo che consentono di adattare la misura in un batter d'occhio.

Le maschere oronasali e facciali per NIV Hamilton Medical sono ora disponibili con una nuova struttura basata su gomiti interscambiabili.
Proponiamo maschere per pazienti pediatrici e adulti. È possibile scegliere tra le maschere per NIV Hamilton Medical con configurazione facciale, oronasale e nasale, disponibili in diverse misure per garantire un'aderenza perfetta a ciascun paziente. Le maschere oronasali e facciali per NIV Hamilton Medical sono ora dotate di una nuova struttura basata su gomiti interscambiabili.
Le maschere oronasali e facciali per NIV Hamilton Medical sono ora dotate di una nuova struttura basata su gomiti interscambiabili. Scegliete l'adattatore ideale in base alle vostre esigenze.
La nostra Academy online propone percorsi di formazione semplici per acquisire familiarità con i prodotti e le tecnologie Hamilton Medical il più rapidamente possibile.

Una panoramica dei benefici e della rilevanza clinica della ventilazione non invasiva accompagnata da consigli pratici per la scelta dell'interfaccia corretta, la regolazione delle impostazioni e il monitoraggio dei pazienti.